Will Canada's Multi-Center Approval Processes Help Your Oncology Clinical Trial Exceed Pivotal Milestones? - Scimega
Doctoral Dissertation Research and the IRB | 2021 | IRB Blog | Institutional Review Board | Teachers College, Columbia University
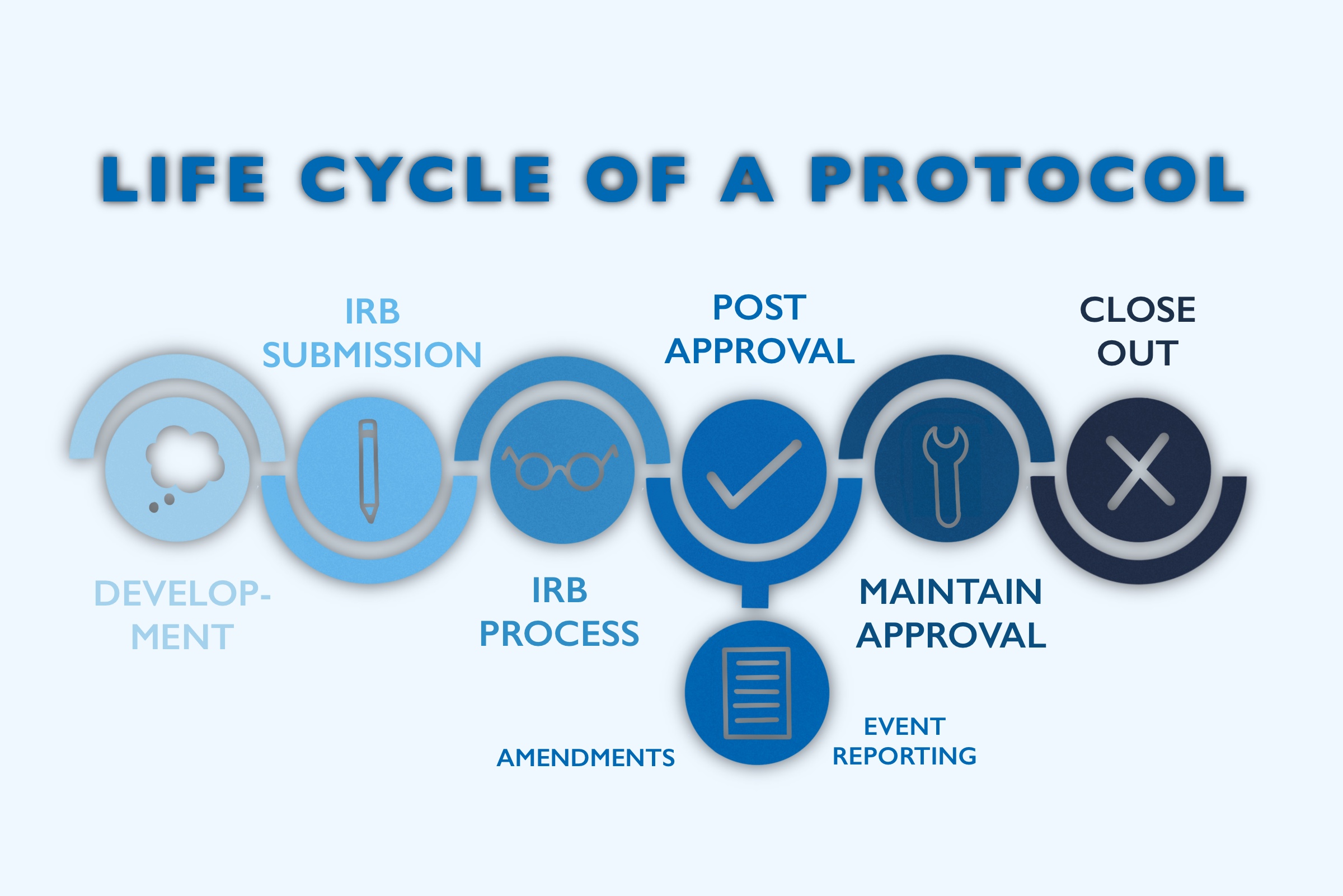
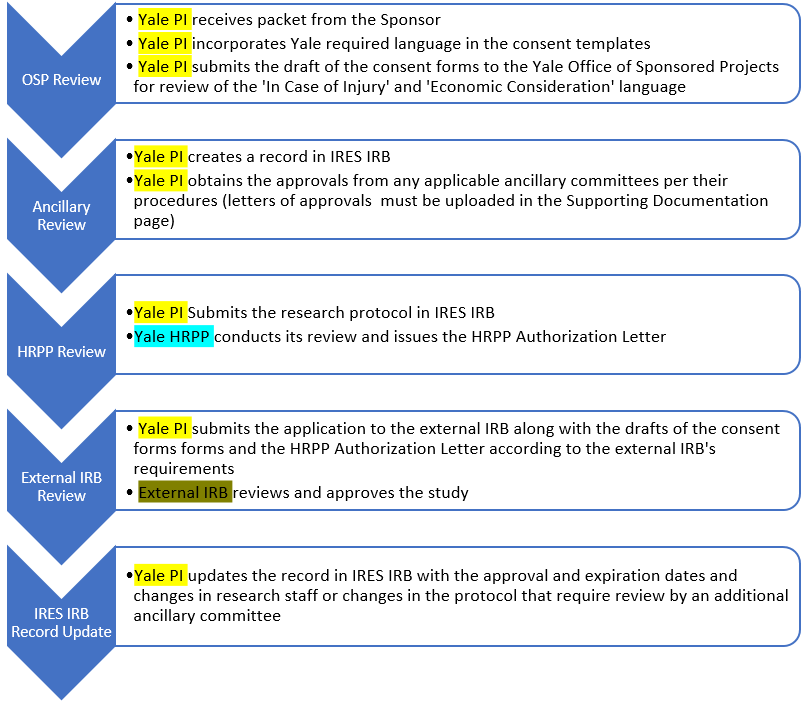
How do Institutional Review Boards (IRB) and Ethics Committees (EC) impact clinical trials? - Clincierge
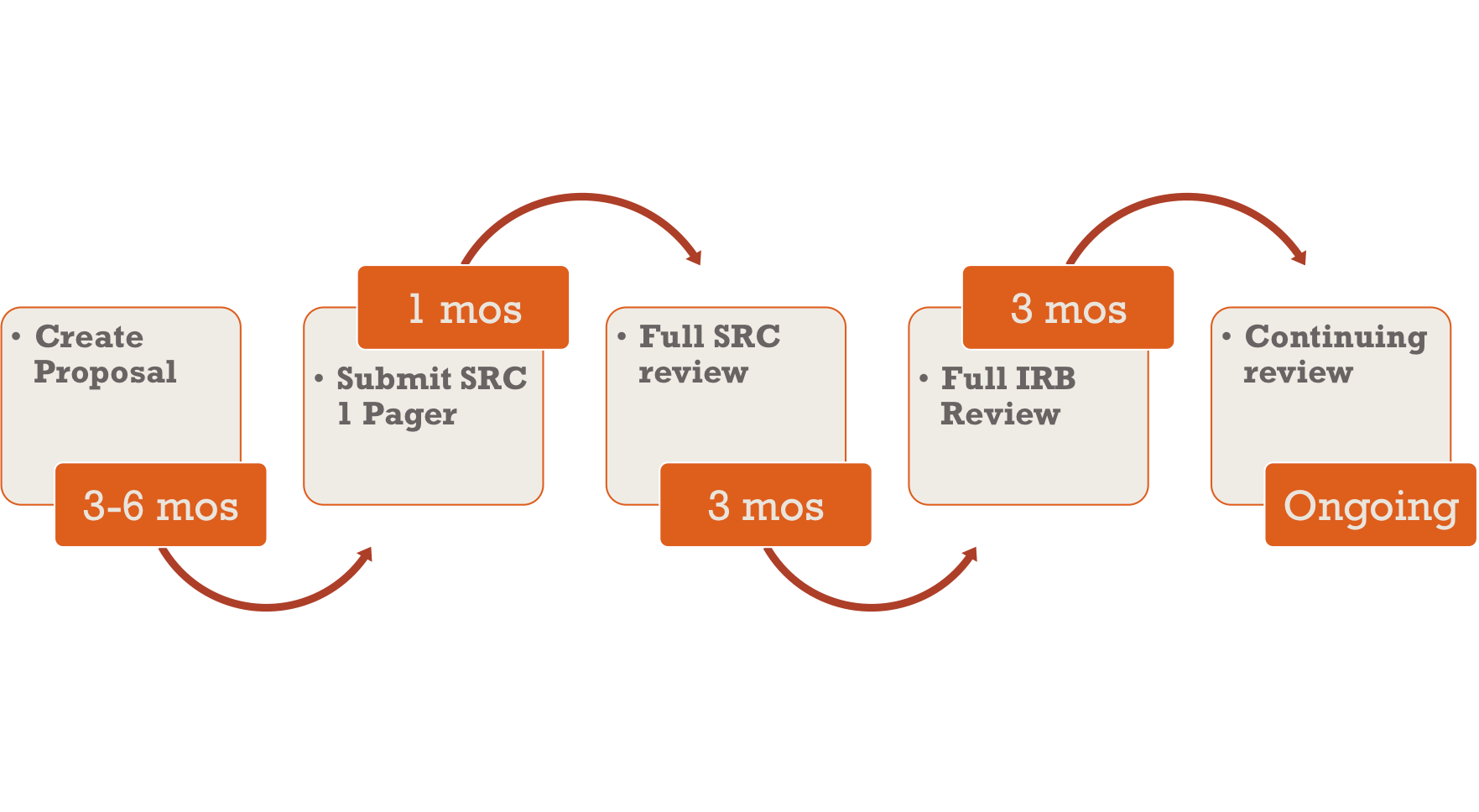
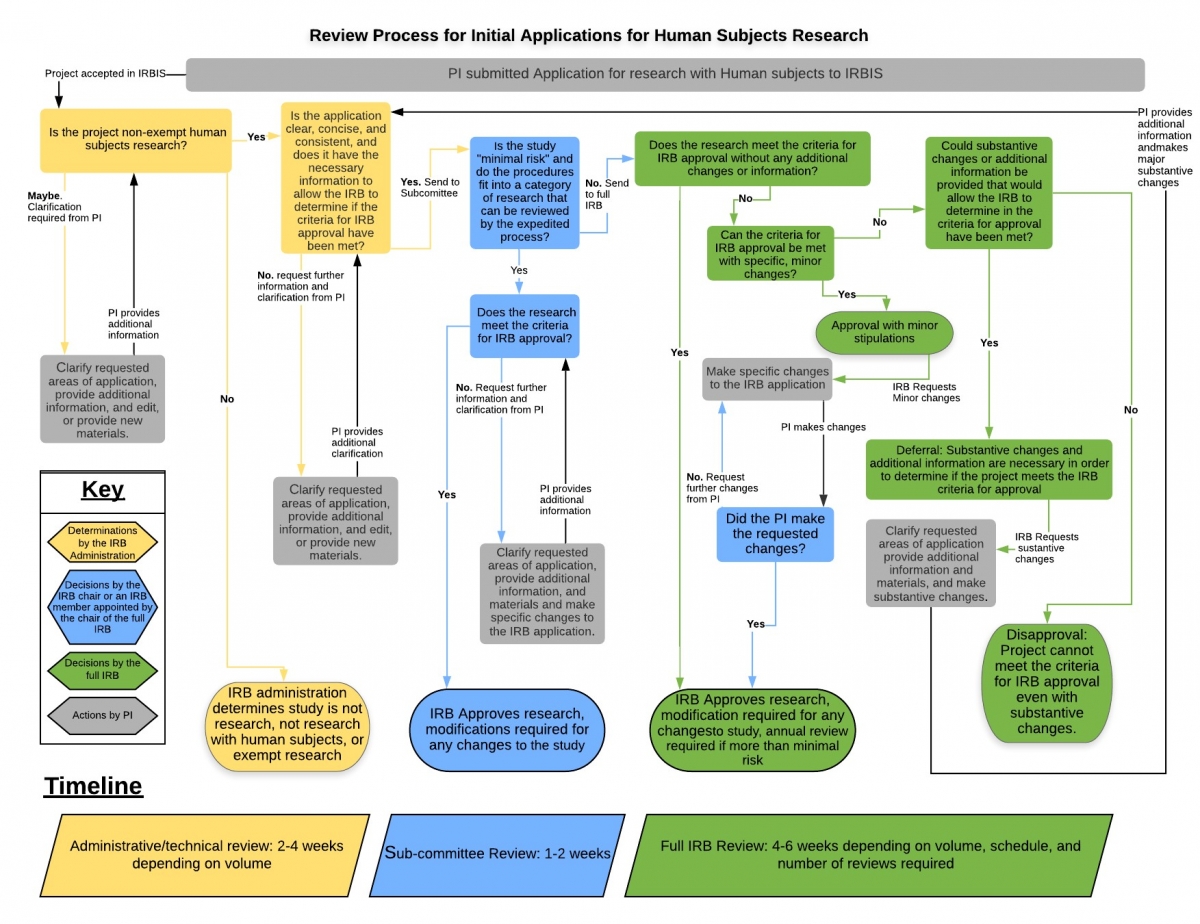
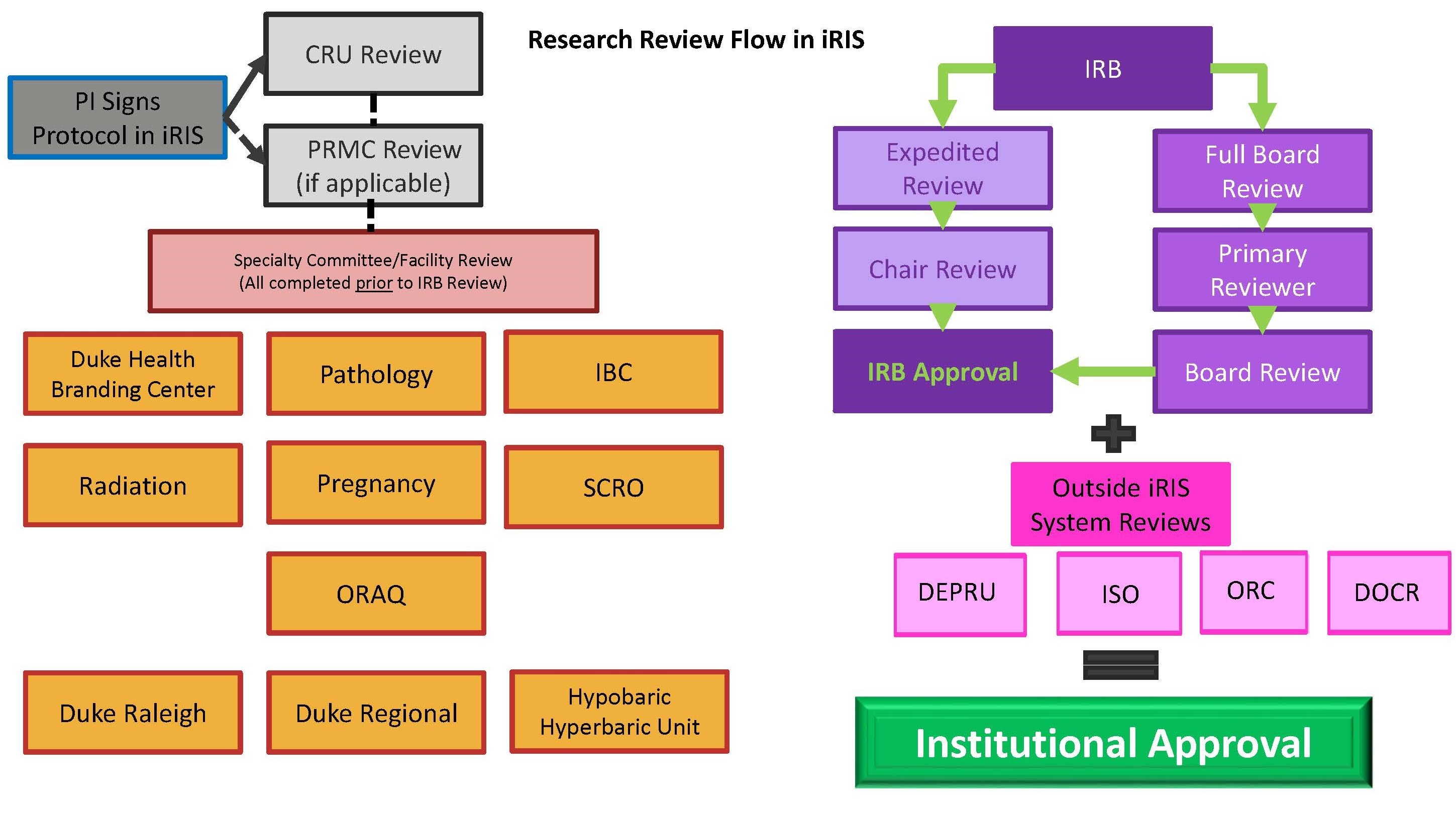
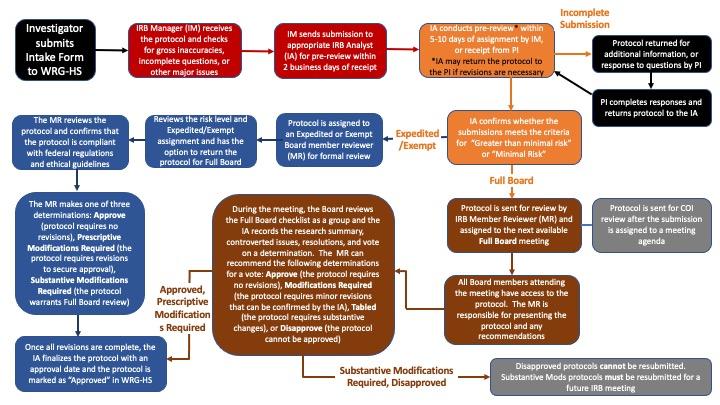
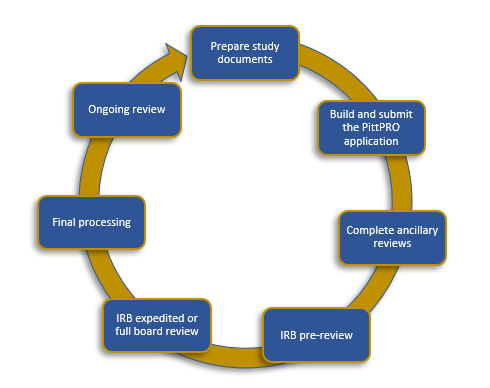
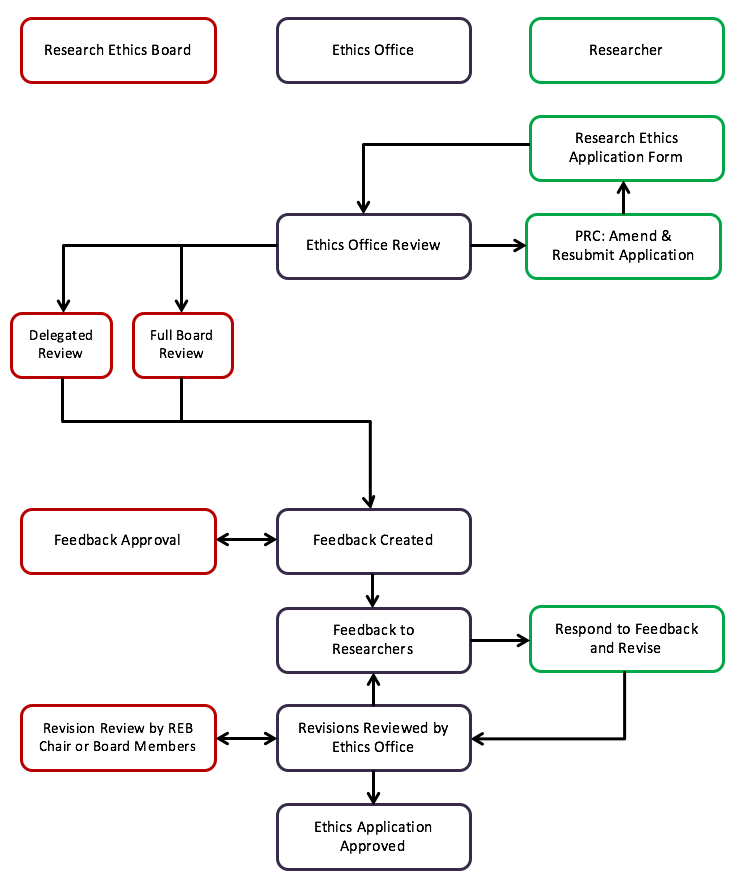
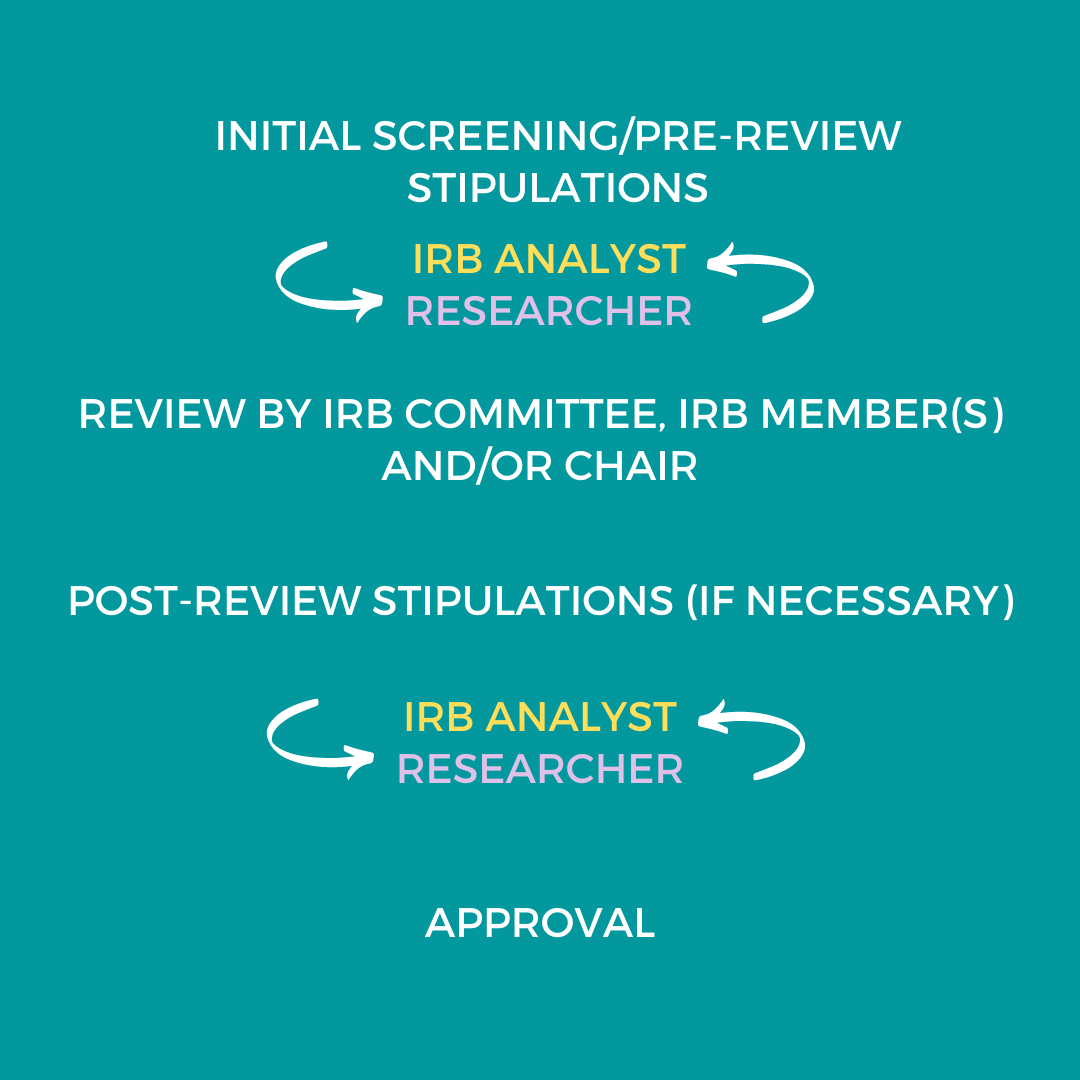
RESEARCH ETHICS BOARD STANDARD OPERATING PROCEDURES SOP Number 404 Ongoing REB Review Activities Date of Issue 2022 07 13 Purp